|
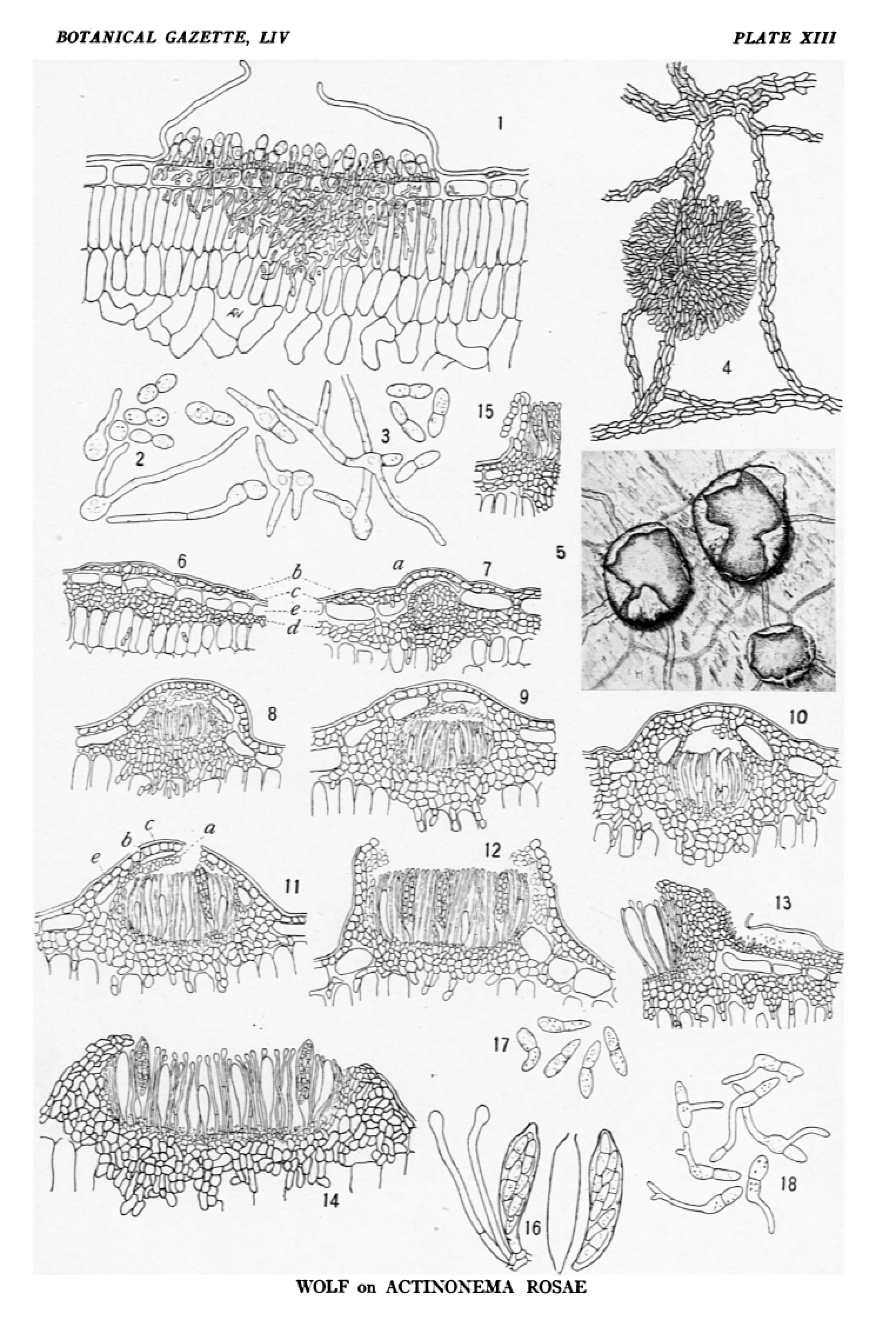
note (WITH PLATE XIII) Perhaps no plant disease has been more widely observed or is more generally known, both in Europe and the United States, than the black spot of roses caused by the parasitic fungus Actinonema rosae (Lib.) Fries. The spots, which are more or less circular in outline, are characterized by a very irregular, fibrillose border. This fibrillose character is due to the radiating strands of mycelium which occur beneath the cuticle. Appearing among the mycelial strands are numerous dark specks, the fruit bodies of the fungus. The spots may be isolated and confluent, or so numerous as to involve the entire upper surface of the leaf. Plants which are attacked become defoliated early in the season, and the leaf buds, which should remain dormant till the next year, often open late in the season. As a result, the plant is weakened so that it blossoms poorly or not at all in the following season. Since very little is known concerning the life history of the fungus and the development of the Actinonema stage, an attempt has been made by cultures on artificial media and on the host to furnish a more satisfactory knowledge of this interesting organism. Before giving an account of this study it may be well to state briefly the characters of the vegetative and fruiting structures of the rose Actinonema. The vegetative body of the fungus consists of two parts, the subcuticular mycelium and the internal mycelium. The subcuticular mycelium is immediately underneath the cuticle, being above the outer wall of the epidermal cells. It consists of branched, radiating strands of mycelium which anastomose, making a network. Each strand consists of several filaments united together, either side by side or sometimes superimposed. At the right of the acervulus in fig.1 is shown a cross-section of one of these strands. The internal mycelium penetrates the mesophyll of the leaf and furnishes nutriment for the subcuticular part. It is connected with the latter by occasional hyphae which penetrate the epidermal cells or pass between them. A section of the fruit bodies or acervuli perpendicular to the surface of the leaf shows that they are formed between the cuticle and the outer wall of the epidermal cells. They are consequently flattened. The stroma of the acervulus is seated directly on the epidermal cells and consists of a very thin layer of small, hyaline to yellowish, pseudoparenchymatous cells. It is connected with the internal mycelium below the hyphae which extend either through or between the epidermal cells into the mesophyll. Laterally the stroma is connected with the subcuticular mycelium. There is no wall or membrane of fungous tissue covering the acervulus. On the upper side of this stroma certain cells are formed which bear the conidia. These conidiophores are not prominently differentiated in form from the other stromatic cells, but are slightly elongated upward. The conidia are hyaline, 2-celled, and oval to elliptical in outline. They are usually somewhat constricted at the septum. The conidia are formed on the somewhat pointed upper ends of the conidiophore layer. The great numbers which are produced cause such a pressure that the cuticle is finally ruptured. The cuticle, which is the only covering for the acervulus, is thus thrown back irregularly, exposing the mass of conidia and permitting their escape. While the spots together with the mycelial strands and acervuli appear dark, this color is not due to the fungus, which is almost colorless, but to the disintegration of the cells below the spot. Development of acervuli It is from the subcuticular mycelium that the acervuli arise. At certain definite points the mycelium begins to form a stroma, which increases in a centrifugal manner, forming a more or less circular stromatic layer. Certain cells of this stroma which are to give rise to the conidia are directed upward as short stalks. These increase in size, forming a closely aggregated layer standing perpendicular to the stroma. Meanwhile, the mesophyll tissue directly below the acervulus is being disintegrated and a dense tangle of fungous filaments is formed in its place. From the perpendicular cells arising from the stroma a cell is cut off by a transverse septum. This cell enlarges into an oval body, the conidium, which soon becomes septate. As the conidia are increasing in size, the pressure on the cuticle above becomes greater and greater, so that it is at length broken, leaving the margin of the exposed acervulus irregularly torn (fig. 1). Sometimes a central papilla is present which marks the place where the cuticle will rupture. At maturity the conidia are oval to elliptical and 2-celled. They are hyaline and 18-25 × 5-6 µ. They may be unequally septate, either straight or subfalcate, and often so deeply constricted at the line of septation that the halves fall apart readily. Several large granules and guttulae are normally present (fig. 3). Germination of conidia The conidia germinate within 24 hours in bean agar or in hanging drops of water. Each of the cells may first enlarge, becoming more or less spherical and vacuolate before the formation of the germ tube. Frequently only one of the cells germinates by the formation of one or two germ tubes (fig. 3). No formation of colonies was secured in poured plates of bean agar, although the fungus grows slowly when the conidia are planted on the surface, forming a small, prostrate, tawny colony. Apparently growth ceases as soon as the reserve food material within the conidium has been utilized in the development of the short hypha. This seems to occur when the hypha is 10-20 times the length of the conidium and may have become branched with several septa. If such conidia are cut out with as little of the surrounding medium as possible and transferred to bean pods, using ordinary sanitary precautions, and if the medium is spread out so as to bring the germinating conidium in contact with the pod, further growth may be induced. In two or three weeks small colonies are formed. At first the mycelium is whitish, changing to a pinkish color and becoming pale brown to blackish with age. The colonies do not spread out on bean pods, but form knots of fungous tissue often one-half as high as the diameter of the colony. The tissue of the bean which is attacked becomes blackened in a fibrillose manner, simulating the blotch on the leaves. Conidia are formed readily on the ends of the hyphae. Such conidia are often so strongly constricted at the septum that each cell is round. There is, then, little surface contact between cells and they are readily separable one from the other. These spherical halves germinate in the normal manner (fig. 2). Acervuli apparently like those on the leaves are also formed on bean pods in the blackened areas. These bear conidia like the typical ones from rose leaves. Systematic position of the conidial stage The genus Actinonema is usually placed by systematists in the Sphaeroidaceae.note This family is a group of imperfect fungi possessing a pycnidium of the type present in Ascochyta, Sphaeropsis, etc. The pycnidium or conceptacle is more or less oval in form, with a membranaceous wall of fungous tissue, usually opening at the apex by a minute pore. Some writers speak of the fruit bodies of Actinonema as pycnidia note or perithecia. note Frank note considered them as very flat spermagonia ('des sehr flachen Spermagoniums"). Sorauer note speaks of them as small astomate pycnidia ("die kleinen mündungslosen Pykniden"). It is very evident from the foregoing account that the conidial stage of the rose Actinonema is not the type in which the conidia are borne in a pycnidium or a perithecium. The conidia are borne in an acervulus resembling that found in Melanconiales, as exampled by Gleosporium, Marsonia, etc. Scribner note has correctly figured the structure of the acervulus and says that while the fungus from analogy is placed with the sphaeriaceous fungi, no perithecia-like or pycnidial structures have been observed. Partly because of the different interpretations of the morphology of this fungus it has been variously names by different workers. In 1849 the name Actinonema rosae (Lib.) Fr.note was employed. In 1853 Bonorden note decribed it as Dicoccum rosae, one of the Hyphomycetes. He says that the fungus forms small, closely aggregated pustules of a brown green color which dehisce irregularly. From collections made in 1888-1889, Briosi and Cavara distributed the species under the name Marsonia rosae (Bon.) Br. & Cav. note because they recognized the acervulus type of fruit body which is characteristic of the Melanconiales. The 2-celled hyaline conidia suggested its position in the genus Marsonia. note I have been able to examine the specimens distributed by Briosi and Cavara and have found them to be the same as rose Actinonema in the United States. The drawing of acervulus and spores which accompany the specimens show the same structure.note Saccardo note notes that Marsonia rosae (Bon.) Br. and Cav. resembles Actinonema rosae (Lib.) Fr. This same fungus was described by Trail note in 1889 as occurring on roses in Scotland. He called it Marsonia rosae. The characters of the genus Actinonema have changed from time to time since the genus was established by Persoon. note He applied the name to those forms on leaves and stems having radiate sterile mycelial strands. He describes two species, A. crataegi and A. caulincola, in neither of which perithecia or conidia were observed. In 1828, Fries note included two species in the genus Actinonema, A. padi, and A. crataegi, the latter showing at length perithecia-like structures, but no conidia were observed. In 1829 note he employed the name Actinonema for the sterile state of fungi belonging to the Pyrenomycetes and Perisporiaceae. Later note he characterized the genus as having a fibrillose, radiating mycelium, a delicate perithecium, and bilocular spores, and lists A. rosae as one of the species which often possesses only a sterile mycelium. Saccardo note employs these characters as given by Fries and notes that the fruits have not been observed in many species. Of the 18 species of Actinonema which have been described, there are 8 species in which the spores were not observed at that time. The radiating fibrillose character of the mycelium has been used as the principal generic character for these species, thus employing the distinctive character as originally given by Persoon. Lindau note includes in Actinonema astomate pycnidial forms occurring on leaves. The pycnidia arise from radiately actinic strands of mycelium. The genus Marsonia is characterized by having subepidermal acervulus, in which are produced hyaline, 2-celled conidia, very similar to the conidia of Actinonema. Several species of Marsonia have been described, however, in which the acervulus is subcutaneous, as Marsonia baptisiae E. & E., M. panatoniana Berl., and M. fructigena (Rick.) Berl. Briosi and Cavara recognized the true morphology of the rose Actinonema acervulus, but attached no significance to the fact that it was subcuticular and not subepidermal. The Actinonema-like character of the mycelium was not taken into account by them as indicative of generic position. Trail must have been of the same opinion when he named this same fungus Marsonia rosae. Even though the subepidermal acervulus has been made one of the generic characters of Marsonia, it would seem that these subcuticular forms might properly be placed in this genus. On the other hand, we do not know the structure of the perithecia or pycnidia of other species of Actinonema. If we accept Persoon's characterization of the genus, it has no fruit bodies, but consists only of sterile mycelium. The rose fungus evidently, then, does not possess the characters of a typical Marsonia, nor does it agree with the original characterization of Actinonema. Whether these differences are worthy of good generic rank, separating it from both these genera, is a matter for consideration. Development of the ascigerous stage During the autumn of 1910, leaves attacked by the conidial stage were collected and placed in wire cages to winter out of doors. When some of these leaves were brought into the laboratory early in April and examined, shield-shaped structures suggestive of the perithecia of the Microthyriaceae were found to be present. At this time, however, no spores had been developed. Fig. 2 shows one of these perithecia as seen in surface view. Such preparations were made by stripping off the epidermis of the leaf together with the perithecia. By April 27 these perithecia had matured and were found to possess characters similar to the genus Asterella, a genus apparently including heterogeneous elements. For the study of the development of the perithecia, material was killed in Merkel's fluid and stained with Flemming's triple stain. By killing material at different times during a period of three weeks, many of the developmental stages were obtained. Not all perithecia on the same leaf are in the same stage of development at the same time. Unfortunately the material was too far advanced for the study of fertilization and the immediate subsequent development. This in itself would be a very interesting study, since nothing is known of these phenomena in the Microthyriaceae. The shield was found to be entirely separate in origin from the tissue which gives rise to the asci. It is formed immediately beneath the cuticle from the radiating strands of mycelium which now are thick-walled and dark brown in color. The strands themselves can be traced across the shield (fig. 4), showing that the growth begins at any point on the mycelial strand and new cells are added in a centrifugal manner. In this way a more or less circular shield is formed, the elements of which are arranged in a radiating manner, especially noticeable at the margin of the shield. The cells which make up the shield possess thick, dark walls. The shield varies in diameter from 100 to 250 µ, and may be more than one cell in thickness. In fig. 6 is shown a young stage in the development of a perithecium. The shield forms a thin layer above the epidermal cells or beneath the elevated cuticle. Beneath the epidermal cells, and above the palisade parenchyma, is an undifferentiated layer of fungous tissue, the stroma from which the asci later arise. This stroma is 3-6 cells in thickness and is made up of cells similar to those of the shield. Occasional filaments connect these two layers through the epidermal cells of the host. In fig. 7, when the fertile layer has increased and the fruit body has begun to be differentiated, the shield is still distinct and not connected with it at the margin. At this time the cells in the center of the young fertile stroma are thinner walled, with a more deeply staining content. The asci are formed within the fertile stroma, arising from the basal portion, as shown in figs. 8 and 9. In this way the cells in the upper part of the fertile stroma persist, forming a delicate covering over the hymenium. The development of the asci within this fertile stroma is comparable with their origin in the apothecia of the Phacidiales. The hymenium arises in the same way, and the upper part of the stroma corresponds with the tissue which covers the hymenium before the opening of the apothecium. In the rose fungus, however, this covering is not so well developed and may not always persist to be folded back when the fruit body opens. It may form a continuous delicate layer over the asci until the fruit body is mature and only rupture together with the shield. In other cases this covering is broken by the development of the hymenial layer. Fragments may remain at the margin of the apothecium or they may disappear. It is only by the elongation of the asci and the consequent increase of pressure that the cuticle and shield, together with the upper part of the apothecium, are ruptured in an irregularly stellate manner and thrown back. The portions covering the hymenium have ruptured in fig. 11. In the mature opened condition shown in fig. 12, the thin-walled cells of the upper part of the apothecium still persist on the margin of the fruit body. The opened perithecia present in surface view the appearance shown in fig. 5. The folding back of the shield is shown in section in fig. 15. The perithecia develop independently of the acervuli, as would be expected from the origin of the two. In fig. 13 is shown an old acervulus by the side of a perithecium. In none of the material which had wintered could acervuli be found which were bearing conidia. The epidermal cells of the host persist for a long time, so that the ascogenous layer and shield are separated. They may become entirely destroyed as the asci elongate and the perithecium becomes mature (fig. 14), or they may persist on the margin of the mature perithecium (fig. 12). The perithecia vary in shape from spherical to discoid. One of the large discoid perithecia is represented in the section in fig. 14. The septate, knobbed paraphyses extend between and beyond the asci until the time when the spores are nearly mature. Asci in many stages of development occur within each perithecium. Mature asci extend slightly beyond the paraphyses and the spores are discharged from an apical pore (fig. 16) formed by the rupture of the wall. The asci are oblong or subclavate, tapering above rather bluntly, and are 70-80×15 µ. Apparently the spores are not discharged with violence. Agar plates were inverted above rose leaves in moist chambers, the surface of the agar coming nearly in contact with the leaf. No spores were observed to have lodged on the surface of the agar, as would be expected if they were projected forcibly from the ascus. As far as I have been able to observe, they merely pile up in a whitish heap in the opened perithecium. The spores are 20-25×5-6 µ, varying extremely in form (fig. 17), as do the conidia. They resemble the conidia very much except that they are not so strongly constricted at the septum. They are hyaline and bicellular. Usually large granules and several guttulae are present in each cell. The cells are generally unequal in size, the upper one being broader. Germination of ascospores Considerable difficulty was experienced in germinating the ascospores. All attempts to employ artificial media have been unsuccessful. Spores from the same preparation have been used in poured and planted plates of bean agar, in hanging drops of water, in similar drops in which has been placed a small piece of green rose leaf, in infusions made by boiling green rose leaves in water, and in drops of water on rose leaves in a moist chamber. In no case has germination been secured in any other way than by the last method. Germination occurs within 24 hours, the larger cell more often germinating, although either cell is capable of germination. A germ tube is characteristically formed at one side near the end of the spore. This hypha soon branches and septa are laid down (fig. 18). Occasionally two tubes are formed from a single cell. In about 35 transfers of spores to bean pods made in aseptic conditions no growth was secured. From these and the foregoing experiments it would seem that the ascospores are dependent on some stimulus of the living plant for germination. There may be some advantage to the parasite in this, since many spores would germinate before the are able to reach a suitable location on the host. Artificial infection Ascospores were used in the infection experiments. Since they are discharged in such masses in the opened perithecia, they can easily be removed free of everything else. Several series of poured plates made from spores obtained in this way remained absolutely sterile, which indicated that no other spores except ascospores of the rose fungus had carried over. The spore masses first removed to a drop of sterile water on a slide. With a needle, then, some of the spores were transferred to drops of water on the leaves of living roses. The plants were then covered with bell-jars and were allowed to remain covered for two days. Infections from inoculations made April 27 were very evident by May 7, appearing as small black areas. By May 15 mature acervuli and conidia of the Actinonema type were formed, thus completing the life cycle and connecting the two forms. Inoculations were also made in the same way on leaves placed in Petri dishes lined with moist filter paper. In four days the radiating strands were very evident with the aid of the low power of a microscope. Infection occurs by the entrance of the germ tube through the cuticle, there being no stomata on the upper surface of the leaves. From the subcuticular mycelium, hyphae later penetrate to the tissue below, first filling the epidermal cells, and only in the advanced stages of the disease penetrating the mesophyll. The way in which this fungus hibernates is no longer a matter of conjecture. Scribner note suggested that the spores lodge on the buds in autumn and remain there dormant until the leaves have expanded the following summer. As has been found to be true with many imperfect fungi, this fungus is carried through the winter on fallen leaves and the ascosporic stage develops in the following spring. This study shows that Gnomoniella rosae (Fkl.) Sacc. is not the perfect stage of the rose Actinonema, as has recently been suggested.note One species of Actinonema, however, has been connected with an Asterella, Actinonema rubi (Fkl.) becoming Asterella rubi (Fkl.) v. Höhnel. note He found in the spring the Asterella stage on living canes of Rubus Idaeus. These areas had been occupied the previous summer by the conidial stage. The genus Asterella was first proposed by Saccardo note as a subgenus of Asterina for those species which have hyaline spores. Later note he raised Asterella to generic rank. As Saccardo himself points out, further investigation of the species which are at present placed in the genus Asterella will result in their transfer to Asterina, since the spores become brown at maturity. Lindau note thinks the existence of this genus is still questionable. Subsequently but little investigation has been made on the genus and no clear-cut generic limits have been proposed. One finds included species whose spores become brown, some which are aparaphysate, some possessing filiform paraphyses, and others having paraphyses which are enlarged at the tips. In fact, the whole family Microthyriaceae is but little known, due in part to the fact that most of the forms are tropical. A thorough investigation of perithecial development is necessary, since very little attention has been given to this group. The family is at present characterized by having perithecia which are shield-shaped, thin membranaceous, flat, with a rounded pore at the top and with a membrane formed only on the upper side. With the exception of the species on rose leaves, which I have studied, it is not known whether or not the forms without an apical pore possess one at maturity. It has long been recognized, because of the entirely different manner of development, that the Microthyriaceae are widely separated from the other two families of the Perisporiales, the Erysiphaceae and the Perisporiaceae. In order to see if other genera of the Microthyriaceae corresponded in structure and development with the forms on rose leaves several of them were examined. Asterina orbicularis B. & C., n. 231 of Ravenel's collections, forms entirely superficial perithecia, sending hyphae partially through the cuticle. Asterina inquinans E. & E., n. 1785 N.A.F., is also superficial, ends of the mycelium being observed in the stomata. Asterina plantaginis Ellis, n. 791 N.A.F., forms spherical perithecia entirely sunken within the host tissue. The perithecia are ostiolate and appear to have the characters of a Sphaerella. Micropeltis longispora Earle, n. 6349, plants of Puerto Rico Porto Rico , is entirely superficial. Microthyrium littigiosum Sacc., collected at Frankfort, Germany, by Dr. Paul Magnus, seems to form superficial perithecia, but the mycelium is present in the epidermal cells. Myriocopron smilacis (De Not.) Sacc., n. 600 E. & E., N.A.F., also forms superficial perithecia and the mycelium occurs in the stomata. None of these representative genera seem to be comparable to the type of development as exhibited by the rose fungus. Since so little is known of the perithecial development and the method of securing food supply of the Microthyriaceae, this family would afford an excellent field for investigation. Maire note has described the organs of absorption of Asterina usterii and Asterina typhospora. A slender filament penetrates the epidermal wall and when it has reached the cavity of the cell it enlarges and becomes profusely branched. The open perithecia of the rose fungus present characters indicative of a close relationship to the Phacidiales. The ragged margin of the shield suggests the ruptured outer portion of the wall of the fruit body which at first covers the hymenium. The presence of knobbed paraphyses is also a character possessed by many Discomycetes. In the Phacidiales, however, as far as can be learned, the upper or outer part of the fruit body is not separate in origin from the ascogenous stroma, nor does it possess the characteristic structure, of the shield present in the Microthyriaceae. On the other hand, it is quite probable that few of the Microthyriaceae possess a stroma within the leaf tissue as has been described for the fungus in question. The majority are apparently superficial and with a well developed wall or shield only on the upper side. In spite of these facts, I feel that this fungus should be placed in the Microthyriaceae. Further morphological study of other species of this genus and related genera will throw some light on the relationship of these microthyriaceous forms. Perhaps the systematic position of many of these forms will be changed as soon as the species have been satisfactorily investigated. While the possession of the shield and the hyaline 2-celled spores are characteristics which would suggest the position of the rose fungus in the genus Asterella, yet, as has been pointed out, this genus is not clearly limited and contains heterogeneous elements. This fungus does not seem to accord morphologically with the members of this genus in the sense in which the genus was first employed. Species representing several generic types apparently have been included in Asterella. Since the characters presented by this fungus are evidently those of a distinct generic type, rather than place it in the genus Asterella, it seems better to treat it as the type of a new genus. Because of the two separate structures, the shield and apothecium, the name Diplocarpon note is proposed. The following description of the genus is given. Diplocarpon, nov. gen.—Fruit bodies formed in connection with an extensive subcuticular mycelium, consisting of a subcuticular circular shield with more or less radiate elements especially at the margin, and an innate apothecium. Shield, together with the radiating strands on which it is formed, dark brown, without a central pore. Apothecium at first separate from the shield, only joined here and there by hyphae which pass between the epidermal cells. Apothecium joined with the margin of the shield at maturity. Hymenium covered by the shield and upper part of the apothecium which at maturity rupture in an irregularly stellate manner. Asci oblong to subclavate, 8-spored; paraphyses unbranched; spores elongated, 2-celled, hyaline at maturity. A conidial stage of the Actinonema-type occurs in one species. Peritheciis scutulum suncutaneum et apothecium innatum constitutis; scutulo mycelio subcutaneo, lato extenso, atro-brunneolo insidiente; margine radialiter diffuso, contextu membraneo, astomate; apothecio innato, primo scutulo separato, maturitate margine adjuncto. Peritheciis centro stellatim laciniato-dehiscentibus; ascis oblongis; paraphysibus simplicibus; sporidiis oblongo-ellipticis, bicellularibus, maturitate hylinis. Actinonema uni speciei cujus statum conidicum sistit. Since this study connects for the first time the ascosporic stage with the conidial stage of the black spot of rose leaves, a brief characterization of the species is added: Diplocarpon rosae, n.n. Syn. Erysiphe radiosum Fr. Observationes Mycologicae. 207. 1824. Asteroma rosae Lib. Mem. Soc. Linn. 5:404-406. 1827. Actinonema rosea Fr. Summa. veg. Scand. 424. 1849. Dicoccum rosae Bon, Bot. Zeit. 282. 1853. Marsonia rosae Trail. Fung. Inverar. 46. 1889. Marsonia rosae Br. and Cav. Funghi parassiti n. 97. 1889. Ascigerous stage.—Perithecia epiphyllous, spherical to disciform, 100-250 µ in diameter; upper part or shield dark brown, subcuticular, formed in conjunction with the radiating strands of mycelium, circular, with a more or less radiating structure toward the margin. Lower part of fruit body disciform, subepidermal, of several layers of pseudoparenchyma cells, the outer of which are dark brown, the margin at length breaking through the epidermis and here and there becoming connected with the margin of the shield. Fruit bodies closed at first, later opening by the rupturing of the shield together with the upper part of the apothecium in an irregularly stellate manner from the center. Asci oblong or subclavate, narrowed abruptly above, 70-80×15 µ, 8-spored; paraphyses slender, enlarged abruptly at the tip, often 1-septate. Spores oblong-elliptical, hyaline, unequally 2-celled, constricted at the septum, 20-25×5-6 µ; upper cell somewhat larger, cells usually guttulate. Conidial stage.—Spots epiphyllous, large, dark brown or blackish, with an irregular radiating border, when numerous becoming confluent and sometimes involving entire leaf. Mycelial strands composed of several filaments, at first hyaline, forming a subcuticular network. Internal mycelium connected through the epidermis with subcuticular mycelium. Acervuli subcutaneous, covered at first by the cuticle which ruptures irregularly; conidia 2-celled, often deeply constricted, straight or subfalcate, 18-25×5-6 µ, hyaline and guttulate. Conidial stage appearing on rose leaves in summer and autumn often causing defoliation of the plants. Ascigerous stage appearing in the spring on fallen leaves which have remained on the ground. Peritheciis epiphyllus, globosis v. disciformibus, 100-250 µ diam.; scutulo atro-brunneolo, subcutaneo, mycelio reticulato insidiente, orbiculare, margine plus minusve radioso. Apothecio primo epidermide tecto, demum margine scutuli adjuncto, in centro irregulari-stellato dehiscente. Ascis oblongis vel subclavatis, supra obtuse angustatis, 70-80×15 µ, octosporis; paraphysibus filiformibus, apice incrassatis, interdum 1-septatis; sporidiis oblongo-ellipticis, inaequaliter bicellularibis, ad septa constrictis, guttulatis, hyalinis, 20-25×5-6 µ. Hab. in foliis dejectis Rosae sp. Status conidicus: Maculis epiphyllis, atro-brunneis vel purpurascentibus, fibrillis e centro radiantibus, albido-arachnoideis; acervulis subcutaneis, sparsis, nigracantibus; conidiis constricto. 1-septatis, guttulatis, hyalinis 18-25×5-6 µ. Hab. in foliis vivis Rosae sp. Susceptibility of the host This disease occurs on nearly all the cultivated varieties of roses both out of doors and in the greenhouse. Briosi and Cavara note that only four varieties, Rosa hybrida var. Belle Angevine, Triomphe d’Aleçon, Abel Grant, Rosa borboniaria var. Triumph d’Anger, of the 600 growing in the botanical gardens at Pavia are free from attacks of this fungus. Laubert and Schwartz note call attention to the fact that the bushy sorts are more susceptible than climbing varieties, also that thin-leaved species are most liable to attack. Halsted note finds that a wild species, Rosa humilis, is also subject to attack when growing in a garden with diseased plants. The amount of loss caused is equaled or surpassed by only one other rose disease, the powdery mildew. Control measures This disease has been very satisfactorily controlled by the use of any of the standard copper compounds. Since now we know that the fungus winters over in the fallen leaves, sanitary measures may better be employed in combating the disease. If all the leaves are gathered together and burned either late in autumn or early in the spring, before the new leaves have expanded, the chances of infection would be greatly lessened. This investigation was undertaken at the suggestion of and under the careful direction of Professor George F. Atkinson, Cornell University, to who I am very grateful for help and criticism. Agricultural Experiment Station Auburn, Alabama Note.—Since this manuscript has been sent to the publishers, I have received type specimens of Asterella rubi, which had been sent to Professor George F. Atkinson, through the courtesy of Professor F. von Höhnel. Because of the fact that Asterella rubi is the first Asterella to be connected with an Actinonema, and is one of the most recently described species of this genus, it is especially important that it be compared morphologically, with the rose fungus. For the study of the structure of the fruit bodies of Asterella rubi the cortex of some of the affected raspberry canes was imbedded in paraffin and sectioned. The perithecia were found to possess a central pore or ostiolum. They are entirely superficial and with a well developed structure only on the upper side. There is no well defined stroma from which the asci arise. By treating small pieces of the cortex with lactic acid the entire shield may be loosened, and can be floated away, thus proving beyond a doubt that this structure is wholly superficial and not subcuticular. Asterella rubi, therefore, conforms to the present concept of the genus Asterella, but is of an entirely different generic type from that represented by Diplocarpon rosae. Explanation of plate XIII FIG. 1.—Acervulus of conidial stage (Actinonema rosae), with a section of one of the radiating strands at the right of the acervulus; ×400. FIG. 2.—Conidia formed free in culture; the two halves are easily separable; germination of the separated cells; ×400. FIG. 3.—Normal conidia from acervuli, and their method of germination; ×400. FIG. 4.—Surface view of Diplocarpon rosae, showing the shield and subcuticular strands from which it developed; ×110. FIG. 5.—Surface view of mature perithecia in which the shield has been ruptured irregularly and folded back; ×55. FIG. 6.—A very young stage in the development of a fruit body in which the shield and the stroma from which the asci are formed are distinct; C, cuticle; B, epidermal cells; D, ascogenous stroma; ×200. FIG. 7.—A stage in which the fruiting part of the perithecium has begun to be differentiated; the shield and ascogenous stroma are separate; A, young apothecium; ×200. FIG. 8.—Differentiation of the asci with the apothecium; ×200. FIG. 9.—Perithecium in which the epidermal cells still persist between the apothecium and shield; the thin-walled cells of the upper part of the apothecium form a covering over the hymenium; ×200. FIG. 10.—Perithecium in about the same stage of development as fig. 9, but the shield and fertile stroma are completely united; ×200. FIG. 11.—Perithecium which is nearly mature; the hymenial covering has broken; A, upper part of apothecium; B, shield; C, cuticle; E, epidermal cells; ×200. FIG. 12.—Mature fruit body of Diplocarpon rosae; some of the cells of the upper part of the apothecium persist at the margin; ×200. FIG. 13.—An old acervulus persisting at the side of a perithecium; ×200. FIG. 14.—A large disciform perithecium; some asci are mature and in others the spores have not yet been formed; the ascogenous stroma and shield have grown together and the epidermal cells have been destroyed; ×200. FIG. 15.—Section through a mature perithecium, showing the manner in which the shield is folded back; ×200. FIG. 16.—Mature asci and paraphyses; the spores have been discharged apically from one ascus; ×400. FIG. 17.—Ascospores of Diplocarpon rosae; ×400. FIG. 18.—Germination of ascospores; ×400. |